Mobile App Development
Medical Website Development
IOS Development
Android Development
Game Development and Design
Interactive Method of Action Animations
Interactive Tradeshow Exhibits
Gamification
Tablet development (IOS, Android)
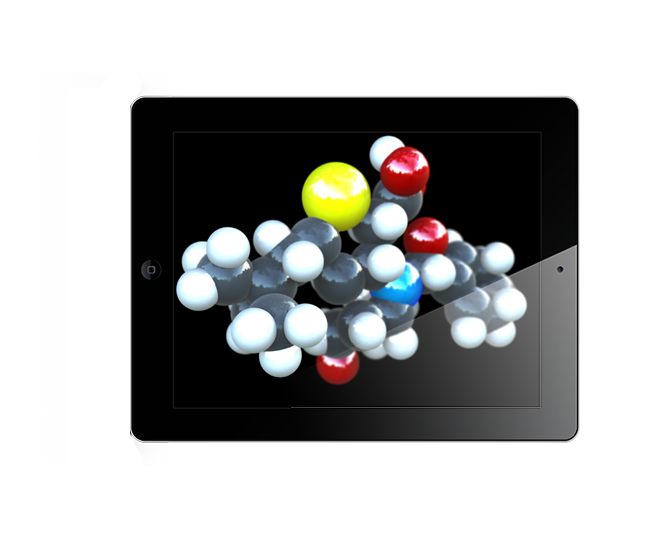
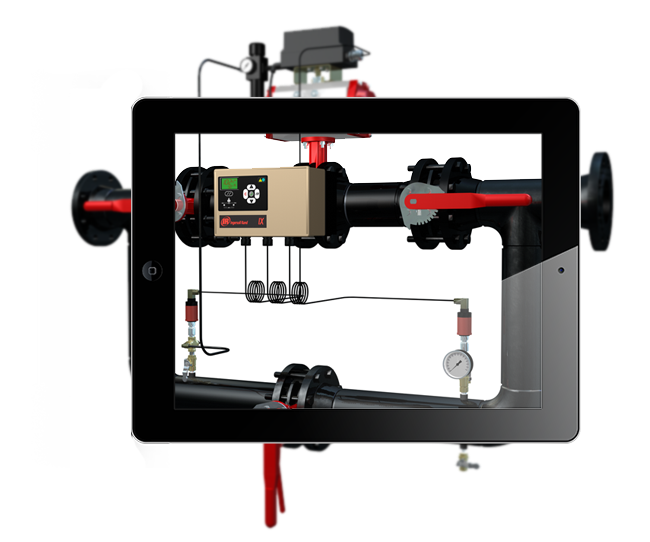
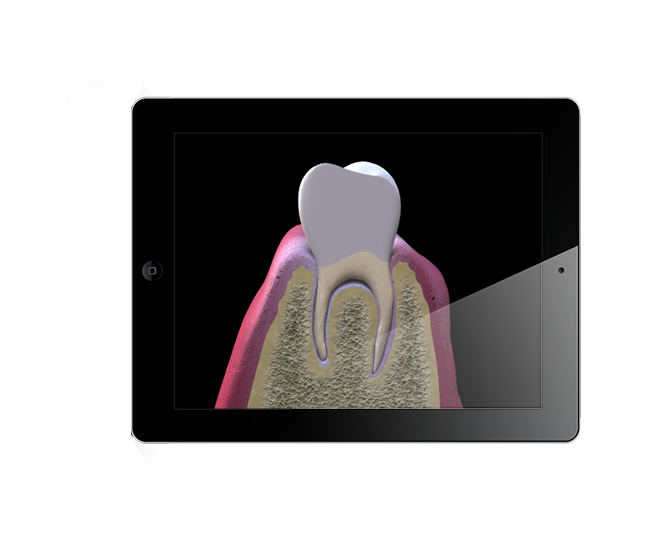
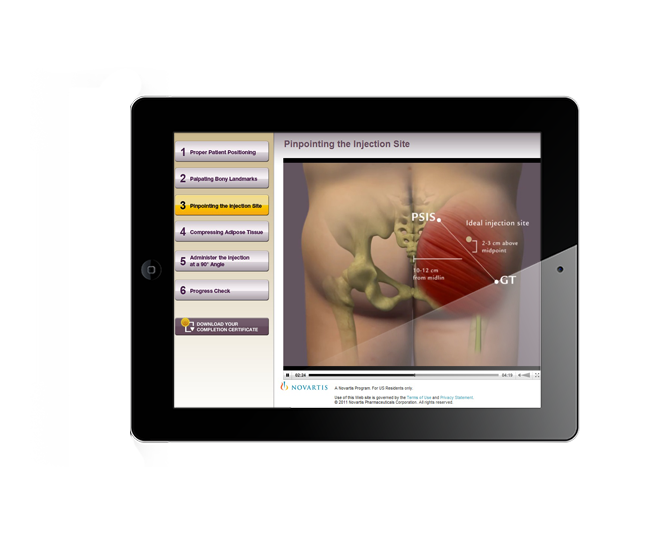
Augmented Reality, Oculus Rift Development
Xbox, Wii, browser and PC gaming development
View Details
Medical Website Development
IOS Development
Android Development
Game Development and Design
Interactive Method of Action Animations
Interactive Tradeshow Exhibits
Gamification
Tablet development (IOS, Android)
Augmented Reality, Oculus Rift Development
Xbox, Wii, browser and PC gaming development
View Details